Traits
Trait: Zinc and insulin secretion (SLC30A8)
Dr Haran Sivapalan
/
August 17, 2021

What is zinc?
Zinc is an essential mineral found in foods such as: oysters, shellfish, beef, beans, and almonds.
The term ‘essential’ means that we need to get zinc from our diet and/or supplements, as we cannot produce it ourselves.
We require zinc because it acts a cofactor for approximately 100 enzymes in the body. Cofactors are helper substances (e.g. metallic ions, such as zinc, or organic molecules such as vitamins) that are needed by enzymes to carry out chemical reactions effectively.
Enzymes that require zinc as a cofactor are involved in several key processes in the body, including:
- Immune function
- Making proteins
- Making DNA
- Cell division
- Wound healing
- Maintenance of nerves
In addition to acting as a cofactor, zinc plays an important role in the production, storage, and secretion of insulin. Accordingly, our intake of zinc can have an effect on our control of our blood sugar levels and our risk of Type II diabetes.
KEY POINTS
- Zinc is an essential mineral required for the production, storage, and secretion of insulin.
What is insulin?
Insulin is a hormone produced by our pancreas that allows glucose (sugar) to be taken up from the bloodstream and be used by cells and tissues.
One popular analogy is that insulin acts like a key that ‘unlocks’ cells, allowing glucose to enter. When insulin (the key) binds to insulin receptors (the lock) on the surface of cells, it ‘opens up’ specialised glucose channels that allow glucose molecules to pass from the bloodstream into cells.

If there are issues with producing and secreting enough insulin, or if cells become less sensitive to the ‘unlocking effects’ of insulin, then it becomes harder for glucose to enter cells. In turn, this can lead to high blood glucose levels, which is damaging to tissue. This is what happens in Type II diabetes, which is characterised by poor insulin function and elevated blood glucose levels.
In addition to facilitating glucose entry into cells, insulin has a number of effects on glucose and fat metabolism. You can read more about these effects of insulin in the Fasting Blood Glucose trait article.
KEY POINTS
- Insulin is a hormone that allows glucose to move into cells and tissues from the bloodstream.
- Insulin is produced by the pancreas.
- Impaired secretion of insulin can lead to high blood glucose levels.
- Poor sensitivity of tissues to the effects of insulin can also lead to high glucose levels.
- High blood glucose levels over time can damage tissues and lead to the development of Type II diabetes.
How is zinc involved in insulin function?
Zinc is involved in the production (synthesis), storage, and secretion of insulin by our pancreas.
Our pancreas have specialised cells known as beta-cells that make insulin from a precursor molecule called proinsulin. Zinc helps to stabilise proinsulin before it is converted into insulin.
Once formed, insulin molecules are then stored in secretory vesicles inside beta cells, ready to be released. Zinc plays a crucial role in condensing insulin molecules into crystals for storage in these secretory vesicles.
When beta cells receive a signal to release insulin (e.g. when glucose levels in the blood rise after eating a meal), the secretory vesicles (also known as ‘insulin granules’) fuse with the membrane and release insulin crystals into the bloodstream.

Source: Kostov, K. (2019). Effects of magnesium deficiency on mechanisms of insulin resistance in type 2 diabetes: focusing on the processes of insulin secretion and signaling. International journal of molecular sciences, 20(6), 1351.
Studies have shown that mice that are deficient in zinc have a lower number of insulin granules in their pancreatic beta-cells and also show impairments in the secretion of insulin. This highlights the importance of zinc in the production, storage, and secretion of insulin by beta-cells.
Zinc may also play a role in the response of other cells and tissues to insulin. When insulin binds to the insulin receptor on a cell, it triggers a complex signalling cascade that results in the opening of glucose transporter channels. Various stages of this insulin signalling cascade are thought to be dependent on zinc.
Supporting this role of zinc in the response to insulin, studies in humans have shown that lower blood zinc levels are associated with poorer insulin sensitivity. Furthermore, people with Type II diabetes are more likely to have lower zinc levels compared to healthy subjects.
As we’ll discuss in the next section, taking zinc supplements may also improve insulin function and control of blood glucose levels, while cutting the risk of developing Type II diabetes.
KEY POINTS
- Zinc is used by beta-cells in the pancreas to produce, store and secrete insulin.
- Zinc helps to condense insulin into crystals for storage.
- Insulin crystals are stored in special secretory vesicles known as insulin granules - this process requires zinc.
- When blood glucose levels rise, insulin granules release insulin into the bloodstream.
- Zinc is also required for insulin to exert its effects on other tissues in the body, when it binds to the insulin receptor.
How does zinc supplementation affect insulin function and Type II diabetes risk?
Several clinical trials have shown that taking zinc supplements can improve markers of insulin function and cut the risk of Type II diabetes.
For example, a meta-analysis of 27 studies found that, compared to placebo, subjects taking low (<25 mg per day) or high (≥25 mg per day) doses of zinc benefitted from significantly healthier markers of insulin function.
These markers included better fasting blood glucose levels, glycated haemoglobin levels (a measure of long term blood sugar control), and HOMA-IR scores (a measure of insulin sensitivity).
Low dose (<25 mg per day) zinc supplementation (as illustrated in the Forest plot below) and long-duration (≥ 12 weeks) supplement regimes were found to have the greatest beneficial effect.

Source: Pompano, L. M., & Boy, E. (2021). Effects of dose and duration of zinc interventions on risk factors for type 2 diabetes and cardiovascular disease: A systematic review and meta-analysis. Advances in Nutrition, 12(1), 141-160.
Other studies have found that zinc supplementation can also reduce the risk of developing Type II diabetes. One prospective study, the Malmö Diet and Cancer Study, followed 26,132 healthy individuals over an average of 19 years and observed how many went on to develop Type II diabetes.
It found that those taking zinc supplements had a 21% lower risk of Type II diabetes.
Interestingly, however, the benefit of taking zinc supplements was shown to vary according to a person’s genetic make-up: specifically, what variants of the SLC30A8 gene they inherited.

Source: Drake, I., Hindy, G., Ericson, U., & Orho-Melander, M. (2017). A prospective study of dietary and supplemental zinc intake and risk of type 2 diabetes depending on genetic variation in SLC30A8. Genes & nutrition, 12(1), 1-11.
While zinc supplementation cut the risk of Type II diabetes irrespective of genotype, as illustrated in the graph above, those with the TT genotype (rs13266634) of the SLC30A8 gene experienced the greatest reduction in risk.
(It's worth noting, however, that the researchers in the above study did not find a significant interaction between zinc supplement use x SLC30A8 genotype).
The influence of the SLC30A8 gene on an individual’s response to zinc supplementation has been found in other studies too. We’ll go into further detail on this in the following sections.
KEY POINTS
- Zinc supplementation can improve insulin function and cut the risk of Type II diabetes.
- The benefits of zinc supplementation / increased zinc levels vary according to genetic factors.
- SLC30A8 gene variants may affect response to zinc supplementation.
What is SLC30A8?
SLC30A8 is a gene that encodes a protein called zinc transporter 8 (ZnT8).
As its name suggests, this transporter protein is responsible for the movement of zinc ions within cells. There are different types of zinc transporter proteins (e.g. ZnT5, ZnT8, ZIP5), which shuttle zinc between different parts of the cell.
ZnT8 in particular transports zinc from the cytoplasm of pancreatic beta-cells into secretory vesicles that contain insulin (so-called insulin granules). As discussed previously, this supports the formation, storage, and secretion of insulin.
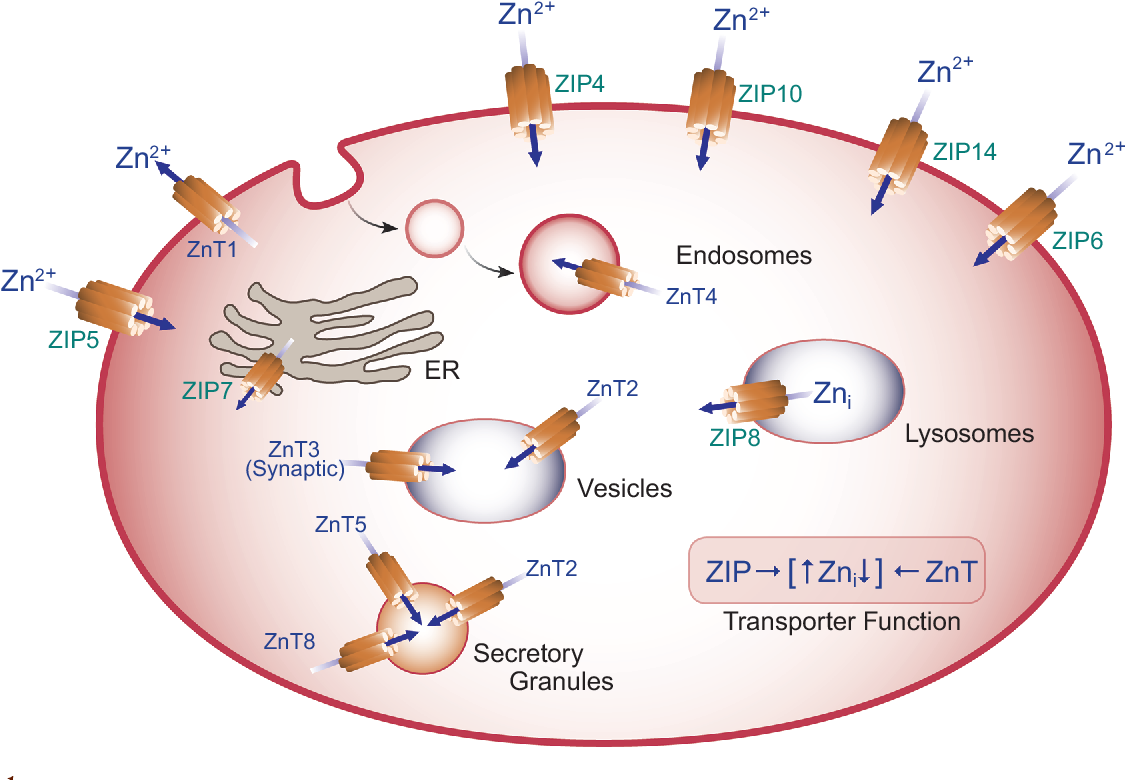
Source: Lichten, L. A., & Cousins, R. J. (2009). Mammalian zinc transporters: nutritional and physiologic regulation. Annual review of nutrition, 29, 153-176.
There are different variants of the SLC30A8 gene, which may affect how beta-cells use zinc and secrete insulin.
On this note, a Single Nucleotide Polymorphism (SNP) within the SLC30A8, designated rs13266634, creates a C --> T change in the DNA code. This gives rise to two different SLC30A8 gene variants or ‘alleles’ – the ‘C’ allele and the ‘T’ allele.
As we inherit alleles in pairs (one from our mother, and one from our father), you may have one of three possible genotypes:
- CC - you carry two copies of the 'C' allele.
- CT - you carry one copy of the 'C' allele and one copy of the 'T' allele.
- TT - you carry two copies of the 'T' allele.
As we’ll explain in the following sections, these different genotypes have been linked to differences in risk of Type II diabetes and response to zinc supplementation.
KEY POINTS
- SLC30A8 encodes zinc transporter 9 (ZnT8) - a transporter protein which moves zinc into vesicles containing insulin.
- ZnT8 allows insulin crystals to be formed and stored, ready for secretion.
- The rs1326634 SNP of the SLC30A8 gene gives rise to two different SLC30A8 gene variants or alleles: 'C' and 'T'.
- The beneficial effects on zinc on inuslin function and risk of Type II diabetes may vary according to what SLC30A8 alleles you inherit.
How do SLC30A8 gene variants affect risk of Type II diabetes?
The ‘C’ allele of the SLC30A8 gene has been linked to a higher risk of Type II diabetes.
A meta-analysis of 46 individual studies (encompassing 71,890 subjects with diabetes and 96,753 healthy controls) found that ‘C’ allele carriers (i.e. those with CC and CT genotypes) had a 14% higher risk of Type II diabetes (after adjusting for age, sex, and BMI). This is illustrated in the Forest plot below.

Source: Fan, M., Li, W., Wang, L., Gu, S., Dong, S., Chen, M., ... & Zheng, C. (2016). Association of SLC30A8 gene polymorphism with type 2 diabetes, evidence from 46 studies: a meta-analysis.
Furthermore, this association between the ‘C’ allele and Type II diabetes was found in European, Asian, and African populations.
When analysed by specific genotype, it was found that those with the CC genotype (i.e. those with two copies of the ‘C’ allele) had the highest risk of Type II diabetes, with a 20% higher risk compared to those with CT and TT genotypes.
Other studies have found that this enhanced risk of Type II diabetes in people with the CC genotype is dependent on a person’s bodyweight. For example, the Malmö Diet and Cancer Study (described in previous sections) found that CC individuals with a healthy BMI (< 25 kg / m2) did not have a higher diabetic risk compared to other genotypes.
By contrast, those who were overweight (BMI 25 – 29.9 kg/m2) and obese (BMI ≥ 30 kg/m2) had a 15% and 31% higher risk of Type II diabetes, respectively. The greater effect of the CC genotype on diabetic risk with higher BMI is illustrated below.

Source: Drake, I., Hindy, G., Ericson, U., & Orho-Melander, M. (2017). A prospective study of dietary and supplemental zinc intake and risk of type 2 diabetes depending on genetic variation in SLC30A8. Genes & nutrition, 12(1), 1-11.
It remains unclear why the ‘C’ allele of the SLC30A8 gene confers a greater risk of Type II diabetes. Some studies suggest that this allele codes for a zinc transporter 8 (ZnT8) protein that has reduced activity and is less effective at transporting zinc into secretory vesicles of pancreatic beta-cells. It is possible that this leads to less efficient secretion of insulin and poorer control of blood glucose levels, which, in turn, can increase the risk of developing diabetes.
KEY POINTS
- The 'C' allele (rs13266634) of the SLC30A8 gene is linked to a higher risk of Type II diabetes.
- The CC genotype is linked to the highest risk of Type II diabetes, although in people who are overweight or obese.
- The 'C' allele may code for a ZnT8 molecule that is less effective at transporting zinc into insulin granules.
How do SLC30A8 gene variants affect response to zinc supplementation?
As explained in a previous section, zinc supplementation has been shown to improve insulin function and reduce the risk of Type II diabetes.
The magnitude of this beneficial response to zinc supplementation, however, depends on what SLC30A8 gene variants you inherit.
Specifically, studies have linked the ‘T’ allele of the SLC30A8 gene with a greater response to zinc supplements.
In the Malmo Diet and Cancer study (described in the “How does zinc supplementation affect insulin function and Type II diabetes risk?” section), the reduction in risk of Type II diabetes was found to be greater for those carrying the ‘T’ allele (i.e. CT/TT genotypes).
Overall, across all genotypes, zinc supplementation was associated with a 21% reduced risk of Type II diabetes. Those with two copies of the ‘T’ allele (i.e. TT genotype), however, experienced a higher risk reduction: a 41% lower risk compared to CC individuals not taking supplements.
The same study also found that, in obese subjects, a higher zinc to iron ratio (an approximate measure of zinc intake) was associated with a significantly greater reduction in diabetic risk for those with the ‘T’ allele (i.e. CT/TT genotypes).
As illustrated in the chart below, the greatest drop in risk of Type II diabetes, when moving from the lowest tertile (T1) to the highest tertile (T3) of zinc to iron ratio, was observed in the TT genotype.

Source: Drake, I., Hindy, G., Ericson, U., & Orho-Melander, M. (2017). A prospective study of dietary and supplemental zinc intake and risk of type 2 diabetes depending on genetic variation in SLC30A8. Genes & nutrition, 12(1), 1-11.
Another study looked at the relationship between SLC30A8 genotype, plasma zinc concentration, and risk of impaired glucose regulation (IGR) and Type II diabetes.
Impaired glucose regulation (often called “impaired glucose tolerance”) refers to a state between healthy blood glucose control and Type II diabetes. It is characterised by blood glucose levels that are elevated, but not high enough to warrant a diagnosis of Type II diabetes.
As can be seen in the bar chart below, higher plasma zinc concentrations (T3 being the tertile with the highest zinc level) were associated with a reduced risk of IGR and Type II diabetes in all genotypes.
The biggest reductions in risk, however, were observed in those with the TT genotype (green bars in the chart).
Furthermore, while not as large as those with the TT genotype, subjects with one copy of the ‘T’ allele (i.e. CT genotype) had a larger risk reduction with increasing zinc intake compared to the CC genotype.

Source: Shan, Z., Bao, W., Zhang, Y., Rong, Y., Wang, X., Jin, Y., ... & Liu, L. (2014). Interactions between zinc transporter-8 gene (SLC30A8) and plasma zinc concentrations for impaired glucose regulation and type 2 diabetes. Diabetes, 63(5), 1796-1803.
It is not completely clear why there is a link between the ‘T’ allele of the SLC30A8 gene (particularly the TT genotype) and a greater benefit of zinc supplements/ higher zinc levels on diabetic risk.
Some studies suggest that zinc supplementation enhances insulin secretion more effectively in ‘T’ allele carriers.
In one study, 57 healthy (non-diabetic) Amish individuals took zinc acetate supplements (containing a total of 100 mg elemental zinc per day) over a period of 14 days. After this treatment period, they then had their insulin levels measured in the first 10 minutes after an intravenous infusion of glucose.
After 5 minutes, those carrying the ‘T’ allele (i.e. CT/TT genotypes) exhibited 26% higher insulin release compared to those with CC genotype. This tentatively suggests that the ‘T’ allele confers a better early insulin response to rises in blood glucose.
KEY POINTS
- The 'T' allele (rs13266634) of the SLC30A8 gene is linked to a greater reduction in risk of Type II diabetes when increasing zinc levels.
- The TT genotype is associated with the greatest benefit of increasing zinc levels.
- 'T' allele carriers (CT and TT genotypes) may secrete insulin more effectively after taking zinc supplements.
How do SLC30A8 gene variants affect response to salt intake?
Some evidence suggests that SLC30A8 genotype can affect response to salt intake.
In one study, researchers genotyped and analysed the dietary patterns of subjects in the Tehran Lipid and Glucose study. Subjects were either healthy controls or had ‘metabolic syndrome’: the name for a collection of risk factors that increase the risk of diabetes and cardiovascular disease.
These risk factors include:
- Abdominal obesity / increased waist circumference
- High blood triglyceride levels (blood fats)
- Low HDL cholesterol levels
- High blood pressure
- High fasting blood glucose level
As illustrated in the graphs below, higher intakes of salty snacks (e.g. crackers, pretzels, chips/crisps, pickles, salted vegetables) were associated with a higher risk of abdominal obesity, but only in subjects carrying the ‘T’ allele (CT/TT genotypes).

Source: Hosseini-Esfahani, F., Mirmiran, P., Koochakpoor, G., Daneshpour, M. S., Guity, K., & Azizi, F. (2017). Some dietary factors can modulate the effect of the zinc transporters 8 polymorphism on the risk of metabolic syndrome. Scientific reports, 7(1), 1-12.
It is unclear why the ‘T’ allele confers greater risk of abdominal obesity with higher salt intake. Speculatively, it may be due to changes in insulin function and fat metabolism in response to consuming salt.
KEY POINTS
- The 'T' allele has been linked to a higher isk of abdominal obesity with increasing salt intake.
How do SLC30A8 gene variants affect response to omega-3 intake?
The CC genotype of the SLC30A8 gene has been associated with a better response to increased intake of omega-3 fatty acids.
An analysis of subjects in the Tehran Lipid and Glucose Study (described in the previous section) found that higher omega-3 intakes were linked to a lower risk of elevated blood glucose levels, high blood triglycerides, and low HDL cholesterol levels.
This effect, however, was only observed in those with the CC genotype, and not in CT or TT genotypes.

Source: Hosseini-Esfahani, F., Mirmiran, P., Koochakpoor, G., Daneshpour, M. S., Guity, K., & Azizi, F. (2017). Some dietary factors can modulate the effect of the zinc transporters 8 polymorphism on the risk of metabolic syndrome. Scientific reports, 7(1), 1-12.
Similarly, higher intakes of fish (which are known to be a rich source of omega-3 fatty acids) were associated with a lower risk of abdominal obesity, but only in those with the CC genotype. This is illustrated in the graph above.
The underlying mechanisms behind this interaction between the CC genotype and omega-3 fatty acid intake are unclear, but it may be due changes in ZnT8 expression in response to polyunsaturated fatty acids.
KEY POINTS
- The CC genotype (two copies of the 'C' allele) has been linked to a lower risk of elevated blood glucose levels, high blood triglycerides, and low HDL cholesterol levels with increasing omega-3 intake.
Your Zinc and insulin secretion (SLC30A8) trait
Your Zinc and insulin secretion (SLC30A8) trait analyses how well you are likely to respond to zinc based on variants of your SLC30A8 gene (specifically the rs13266634 SNP), as well as your lifestyle survey data.
Depending on your results, you will be classified into one of four groups:
- Less responsive to zinc – you have the CC genotype of the SLC30A8 gene linked to lower improvements in diabetic risk and insulin function when increasing your zinc intake.
- Responsive to zinc – you have one copy of the ‘T’ allele (i.e. CT genotype), which is linked to greater improvements in diabetic risk and insulin function when increasing your zinc intake. These improvements, however, are not as high as those expected in people with the TT genotype.
- Enhanced response due to lifestyle - you have one copy of the ‘T’ allele (i.e. CT genotype), which is linked to greater improvements in diabetic risk and insulin function when increasing your zinc intake. Your lifestyle data also suggest you are overweight/obese, which is shown to enhance the benefits of increased zinc intake.
- Enhanced response to zinc – you have the TT genotype linked to the greatest improvements in diabetic risk and insulin function when increasing your zinc intake. Zinc supplementation may be particularly beneficial for people with your genotype.
To find out your result, please login to Truefeed.




.png)
