Traits
Trait: Alcohol metabolism (ALDH2) and health
Dr Haran Sivapalan
/
July 19, 2021

How do we break down alcohol?
After we consume an alcoholic drink, the alcohol (or ethanol – the type of alcohol found in beverages) eventually gets absorbed into the bloodstream, primarily in the small intestine.
From there, it travels to the liver via the portal vein (a major blood vessel that carries blood from the digestive tract to the liver).
The liver is the main site where alcohol is broken down or ‘metabolised’, accounting for 90% of alcohol breakdown. The stomach and brain also produce enzymes that break down alcohol, although they play a relatively minor role compared to the liver.
Alcohol metabolism by the liver
Alcohol is broken down by the liver in two key stages:
1) Alcohol is broken down by various liver enzymes into the intermediate molecule: acetaldehyde.
2) Acetaldehyde is further broken down by into acetate.
Once formed in the liver, acetate re-enters the peripheral bloodstream where it is largely converted into carbon dioxide and water by cells in the brain, heart, and skeletal muscle.
Let’s take a closer look at the two stages of alcohol metabolism in the liver.
Source: Zakhari, S. (2006). Overview: how is alcohol metabolized by the body?. Alcohol research & health, 29(4), 245.
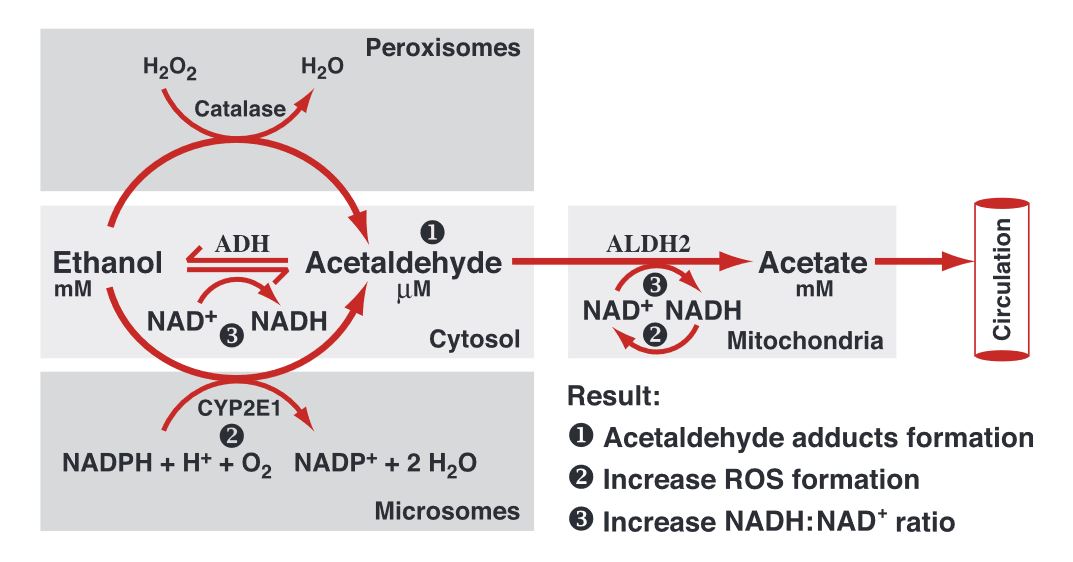
- First stage of alcohol breakdown
Alcohol can be broken down into acetaldehyde using one of three different enzymes: ADH (alcohol dehydrogenase), catalase, and cytochrome P450 enzymes (e.g. CYP2E1).
Of these three enzymes, ADH has the highest activity and plays the biggest role in breaking down alcohol.
- Second stage of alcohol breakdown
Acetaldehyde formed from the first stage of metabolism also needs to be further broken down. This is particularly important because, as explained in the following section, acetaldehyde is a highly reactive and potentially damaging substance.
The enzyme that breaks down acetaldehyde is called ALDH (aldehyde dehydrogenase).
There are two key forms of this enzyme, ALDH1, which is present in the cytoplasm of liver cells, and ALDH2, which is present in the mitochondria of liver cells.
ALDH2 is the main enzyme form that breaks down acetaldehyde, forming two products: acetate and NADH.
As mentioned earlier, acetate re-enters the bloodstream and is converted to carbon dioxide and water, which are then excreted. NADH is further metabolised by mitochondria in a process known as the electron transfer chain.
Rate of alcohol breakdown
The rate at which the ADH and ALDH2 enzymes break down alcohol has a significant influence on our blood alcohol concentration. Generally speaking, alcohol is removed from the bloodstream at a rate of 3.3 mmol per hour (15 mg per 100 ml per hour). Slower activity of the ADH and ALDH2 enzymes will reduce this rate of removal and lead to higher blood alcohol concentrations.

Source: Paton, A. (2005). Alcohol in the body. British Medical Journal, 330(7482), 85-87.
Furthermore, the activity of ADH and ALDH2 enzymes also affects levels of acetaldehyde in the body, and how quickly it accumulates after consuming alcohol.
Higher activity of the ADH enzyme (responsible for the first stage of alcohol metabolism) will cause alcohol to be converted into acetaldehyde more quickly, causing acetaldehyde levels to rise more abruptly.
By contrast, lower activity of the ALDH2 activity (responsible for the second stage of alcohol metabolism) will cause acetaldehyde to be broken down more slowly, leading to the build-up of acetaldehyde in the body.
Lots of different factors affect the activity of ADH and ALDH2 enzymes and the speed at which we metabolise alcohol. One important factor is our history of alcohol consumption. Heavy drinkers show a compensatory increase in ADH and ALDH2 activity in response to high intake of alcohol in the long term.
Another key factor is our genetics, including the genes that affect the activity of ADH and ALDH2. In this trait, we look at gene variants that alter the activity of ALDH2.
KEY POINTS
- Alcohol is broken down in two main stages by liver enzymes.
- In the first stage, alcohol is broken down into acetaldehyde.
- In the second stage, acetaldehyde is broken down into acetate.
- ALDH2 (Aldehyde dehydrogenase 2) is the main liver enzyme responsible for the second stage of alcohol breakdown.
Why is acetaldehyde considered toxic?
Acetaldehyde, formed from the breakdown of alcohol by ALDH2, is a highly reactive compound. Consequently, it can easily react with and damage important molecules in the body, including: membrane proteins, DNA, haemoglobin (the oxygen-carrying pigment in red blood cells), collagen, neurotransmitters in the brain, and various key enzymes.
When acetaldehyde reacts with these molecules, it typically forms a larger joint molecule known as an adduct. Many of these acetaldehyde adducts have damaging effects in the body.
For example, acetaldehyde can bind with DNA to form a carcinogenic adduct called 1,N-propanodeoxyguanosine. Acetaldehyde can also form an adduct with the neurotransmitter dopamine, called salsolinol, which is thought to underlie the addictive properties of alcohol.
In the liver, the formation of acetaldehyde-protein adducts can activate the immune system causing inflammation and immune-related damage of liver cells.

Source: Setshedi, M., Wands, J. R., & de la Monte, S. M. (2010). Acetaldehyde adducts in alcoholic liver disease. Oxidative medicine and cellular longevity, 3(3), 178-185.
Given these damaging effects, it’s thought that higher levels of acetaldehyde may partly underlie the development of alcohol-related cancers such as oesophageal and oropharyngeal cancers.
The diagram above demonstrates a proposed mechanism by which hepatocellular carcinoma (HHC) – a type of liver cancer – may result from elevated acetaldehyde levels over time from chronically high alcohol intake.
KEY POINTS
- Acetaldehyde is highly reactive and capable to binding to and damaging various key molecules e.g. DNA, proteins.
- When acetaldehyde binds to DNA and various proteins, it forms larger toxic molecules known as adducts.
- Acetaldehyde adducts can promote cells processes that lead to cancer.
- High levels of acetaldehyde due to long-term, excessive alcohol consumption can lead to cancer.
What is the ALDH2 gene?
The ALDH2 (aldehyde dehydrogenase 2) enzyme, which is responsible for breaking down acetaldehyde, is coded for by the ALDH2 gene.
Well studied variants of this gene have been shown to affect ALDH2 enzyme activity and, therefore, the rate at which we metabolise alcohol and break down acetaldehyde.
A single nucleotide polymorphism (SNP) within the ALDH2 gene, designated rs671, causes a G > A change in the DNA sequence. This gives rise to two different variants or alleles:
- ‘G’ or ALDH2*1 allele – which codes for an ALDH2 enzyme with normal activity.
- ‘A’ or ALDH2*2 allele – which codes for an ALDH2 enzyme with virtually no activity.
People who inherit the ‘A’ or ALDH2*2 allele therefore have reduced ALDH2 enzyme activity and break down acetaldehyde less effectively after drinking alcohol.
Interestingly, the frequency of the ‘A’ allele differs between ethnic populations. It is virtually absent in European and African populations, but found in much higher frequencies in East Asian populations.
One review of the literature found that roughly a third of Han Chinese individuals, between 41-52% of Japanese, 29-37% of Korean, and 10% of Thai populations carry at least one copy of the ‘A’ allele.
KEY POINTS
- The ALDH2 enzyme is coded for the by ALDH2 gene.
- The 'A' allele (rs671) of the ALDH2 gene codes for an enzyme with virtually no ALDH2 enzyme activity.
- The 'A' allele is more common in East Asian populations and virtually absent in European and African populations.
How do ALDH2 gene variants affect how we break down alcohol?
As explained in the previous section, the ‘A’ (rs671) or ALDH2*2 allele of the ALDH2 gene codes for an ALDH2 enzyme with significantly reduced activity. This leads to lower rates of alcohol breakdown and higher levels of acetaldehyde when alcohol is consumed.
Studies have shown that people who inherit one copy of the ‘A’ allele (i.e. GA genotype) produce an ALDH2 enzyme with only 17-38% of normal activity.
Those who inherit two copies of the ‘A’ / ALDH2 allele (i.e. those with the AA genotype) produce an ALDH2 enzyme that is completely inactive (0% of normal activity).
Consequently, people with one or, especially, two copies of the ‘A’ allele experience much greater rises in acetaldehyde levels after drinking alcohol. On this note, studies suggest that ‘A’ allele carriers may have 6-19 times higher blood levels of acetaldehyde after drinking.

Source: Chang, C. Say No to Glow: Reducing the Carcinogenic Effects of ALDH2 Deficiency.
In one study, 68 Japanese men consumed 0.4 grams of alcohol per kg bodyweight over 10 minutes. Their blood levels of acetaldehyde were then measured at regular intervals over the next 4 hours.
Those who didn’t carry the ‘A’ allele (GG genotype) had an average peak acetaldehyde level of 4.1 micromoles. By contrast, those with. one copy of the ‘A’ allele (GA genotype) had higher acetaldehyde levels: on average, 23.4 micromoles. Those with two copies of the ‘A’ allele (AA genotype) had even an higher average blood acetaldehyde level: 79.3 micromoles.
As well as slowing down the breakdown of acetaldehyde into acetate (i.e. the second stage of alcohol metabolism), reduced ALDH2 enzyme activity in ‘A’ allele carriers is also thought to cause a bottleneck, thereby also slowing down the first stage of alcohol metabolism i.e. the breakdown of alcohol into acetaldehyde.
As acetaldehyde is a toxic intermediate molecule, elevated acetaldehyde levels in ‘A’ allele carriers can contribute to various health issues when consuming alcohol, including unpleasant symptoms of alcohol sensitivity (such as facial flushing) and an increased risk of alcohol-related cancers.
KEY POINTS
- Due to lower ALDH2 activity, people who carry the 'A' allele accumulate acetaldehyde after drinking alcohol.
- People with two copies of the 'A' allele (AA genotype) have virtually zero ALDH2 enzyme activity and experience the greatest rises in acetaldehyde levels after drinking alcohol.
- People with one copy of the 'A' allele (GA genotype) have significantly reduced ALDH2 enzyme activity and also experience rises in acetaldehyde levels after drinking alcohol.
How do ALDH2 gene variants affect alcohol sensitivity?
Some people experience unpleasant symptoms after drinking alcohol, including:
- Facial flushing
- Nausea
- Heart palpitations
- Stuffy nose
These symptoms, sometimes collectively referred to as alcohol sensitivity or alcohol intolerance, result from the build-up of high levels of acetaldehyde after consuming alcohol.
On this note, people who carry the ‘A’ allele of the ALDH2 gene are more likely to have unpleasant symptoms of alcohol sensitivity, due to lower ALDH2 activity and the accumulation of acetaldehyde after drinking alcohol.
Acetaldehyde is thought to stimulate the release of various vasoactive substances, such as histamine and adrenaline, which cause changes in heart rate and blood flow responsible for some of the alcohol sensitivity symptoms. For example, the release of histamine can cause dilation of blood vessels supplying the face, leading to facial flushing and redness after drinking (see photo below).

This facial flushing phenomenon is sometimes known as the ‘Asian flush’ or ‘Asian glow’ as it frequently seen in East Asian populations.
There’s a reason for this: the ‘A’ (rs617) or ALDH2*2 allele is much more common in East Asian populations with roughly a third of Han Chinese individuals, between 41-52% of Japanese, 29-37% of Korean, and 10% of Thai populations thought to carry at least one copy of the ‘A’ allele.
Similarly, if you do experience facial flushing after consuming alcohol, it’s highly likely you carry the ‘A’ allele of the ALDH2 gene. Studies of Japanese men and women have found that facial flushing has an approximately 90% sensitivity for carrying the ‘A’ allele. This means that, of a group of people carrying the ‘A’ allele, 90% experienced facial flushing immediately after drinking a glass (about 180ml) beer.
KEY POINTS
- People with the 'A' allele of the ALDH2 gene are more likely to experience facial flushing ('Asian flush'), nausea, and other unpleasant symptoms after consuming alcohol.
- This is due to the build up of acetaldehyde soon after alcohol is consumed.
How do ALDH2 gene variants affect alcohol consumption?
It’s been widely shown that ‘A’ (ALDH2*2) allele carriers consume less alcohol and are less likely to develop alcohol dependence compared to non-carriers.
This is likely due to the unpleasant symptoms of high acetaldehyde levels (including facial flushing, nausea, more sever hangovers) after consuming alcohol, which deter further drinking.
On this note, an analysis of 2,011 subjects enrolled in the Korean Genome and Epidemiology Study found that those who carried the ‘A’ allele of the ALDH2 gene consumed 7.94 grams of alcohol per day less than non-carriers.
To put this figure in context, a standard drink by US criteria (e.g. a 350 ml can of 5% beer) contains 14 grams of alcohol. In the UK, 1 unit of alcohol (about half a pint of low-strength lager) is equivalent to 8 grams of alcohol.

Perhaps due to lower habitual alcohol intake and unpleasant symptoms following alcohol consumption that deter drinking, ‘A’ allele carriers may also be at reduced risk of developing unhealthy drinking patterns.
One meta-analysis pooled the findings of 53 studies, largely and compared the ALDH2 genotypes of people with alcohol abuse or alcohol dependence to healthy controls (free from alcohol issues).
It found that, among the largely East Asian study subjects, people with alcohol abuse/ dependence were 77% less likely to carry the ‘A’ allele. Furthermore, virtually nobody with two copies of the ‘A’ allele (AA genotype) was found among cases with alcohol abuse/dependence.
KEY POINTS
- 'A' allele carriers are shown to consume less alcohol compared to non-carriers.
- This may be due to unpleasant symptoms of alcohol sensitivity (e.g. flushing, nausea) that deter drinking.
- 'A' allele carriers are also less likely to develop alcohol abuse and alcohol dependence.
How do ALDH2 gene variants affect cancer risk?
People who carry the ‘A’ or ALDH2*2 allele of the ALDH2 gene may be at an increased risk of certain alcohol-related cancers. This may be partly due to elevated levels of acetaldehyde following alcohol consumption, as acetaldehyde can damage DNA and form carcinogenic adducts in the body.
A 2015 meta-analysis of 51 case-control studies in largely East Asian populations found that the ‘A’-allele was associated with a 20% increased cancer risk.
When the analysis focussed on specific types of cancer, the ‘A’ allele was linked to a 52% increased risk of oesophageal cancer, 22% higher risk of head and neck cancer, and an 18% increased risk of gastric cancer.
It’s worth pointing out that the above meta-analysis did not stratify its subjects by alcohol intake (e.g. comparing drinkers to non-drinkers), so it was unable to investigate whether the increased cancer risk in ‘A’ allele carriers was related to alcohol intake.
Given that high levels of acetaldehyde, which can generate carcinogenic adducts in the body, is generated in ‘A’ allele carriers in response to drinking alcohol, it seems likely that the ‘A’ allele confers cancer risk only when combined with alcohol intake.
A meta-analysis of 31 studies into oesophageal cancer supports this, with ‘A’ allele carriers only having increased cancer risk when consuming alcohol. Furthermore, the more alcohol ‘A’ allele carriers consumed, the higher their risk of oesophageal cancer.
In the study, ‘A’ allele carriers who were light drinkers (classified as consuming 1-350 g of alcohol per week) had a 3.8 times greater risk of cancer compared to light-drinking non-carriers. This risk increased for heavy drinkers (classified as consuming >350g of alcohol per week), with ‘A’ allele carriers having a 6.50 greater risk of oesophageal cancer than non-carriers.
KEY POINTS
- 'A' allele carriers are shown to be at an increased risk of oesophageal, gastric, and head and neck cancer.
- This increased cancer risk is dependent on alcohol intake - 'A' allele carriers had a higher cancer risk only when consuming alcohol.
- Increased cancer risk is likely due in part to higher levels of acetaldehyde when drinking alcohol (acetaldehyde can form carcinogenic adducts in the body).
How do ALDH2 gene variants affect other health outcomes?
There is some evidence to suggest that the ‘A’ or ALDH2*2 allele of the ALDH2 gene is protective against hypertension (high blood pressure).
A meta-analysis of 30 studies found that the ‘A’ allele was associated with a 21% lower risk of essential hypertension. This is illustrated in the Forest plot below.

Source: Zheng, Y., Ning, C., Zhang, X., Zhao, Y., Li, Y., Qian, L., ... & Fang, Z. (2020). Association Between ALDH-2 rs671 and Essential Hypertension Risk or Blood Pressure Levels: A Systematic Review and Meta-Analysis. Frontiers in Genetics, 11, 685.
The study also reported that ‘A’ allele carriers had lower systolic and diastolic blood pressure measurements compared to carriers. Furthermore, the ‘A’ allele was found to be particularly protective against high blood pressure in drinkers, an effect thought to be due to lower overall alcohol intake in ‘A’ allele carriers.
KEY POINTS
- 'A' allele carriers may have a reduced risk of high blood pressure (hypertension) compared to non-carriers.
Your Alcohol metabolism (ALDH2) and health trait
Your Alcohol metabolism (ALDH2) and health trait looks at the rs671 SNP in the ALDH2 gene. Depending on your genetic data, you will be classified into one of three groups:
- Reduced ALDH2 activity - you carry two copies of the 'A' allele (AA genotype) leading to virtually no ALDH2 activity. This may cause the build-up of acetaldehyde after consuming alcohol, which can cause facial flushing, alcohol sensitivity, and increased cancer risk. Your genotype (AA) is more commonly seen in East Asian populations and virtually absent in European and African populations.
- Moderately reduced ALDH2 activity - you carry one copy of the 'A' allele (GA genotype) leading to significantly reduced (17-38%) ALDH2 activity. This may cause the build-up of acetaldehyde after consuming alcohol, which can cause facial flushing, alcohol sensitivity, and increased cancer risk. Your genotype (AA) is more commonly seen in East Asian populations and virtually absent in European and African populations.
- Normal ALDH2 activity - you do not carry the 'A' allele (GG genotype) and have normal ALDH2 activity. You are less likely to experience high levels of acetaldehyde immeditately after consuming alcohol. If you are of European or African ancestry, you will most likely fall into this category.
To view your trait result, please login to Truefeed.




.png)
