Traits
Trait: Lactose tolerance
Dr Haran Sivapalan
/
September 20, 2022

What is lactose?
Lactose is the main carbohydrate (or sugar) found in cow’s milk and dairy products.
It is found in the milk of virtually all mammals, including humans, and forms a key source of energy in nursing mammals. Human milk contains about 7% lactose by weight, whereas cow’s milk contains 4-5% lactose by weight.
As infants, we all produce an enzyme (called lactase) that enables us to break down and derive chemical energy from lactose. After weaning, however, the majority of us stop producing this enzyme, which can, in some cases, result in an inability to digest lactose as an adult.

In terms of its chemical structure, lactose is a disaccharide - meaning it is composed of two simple sugars (monosaccharides) linked together. The two simple sugars that make up lactose are glucose and galactose.
KEY POINTS
- Lactose is the main sugar found in cow’s milk and dairy products.
What is lactase?
Lactase, or, more precisely, lactase-phlorizin hydrolase (LPH) is an enzyme that breaks down lactose.
Cells that line our small intestine, known as intestinal epithelial cells, are the principal producers of lactase. These cells have finger- or hair-like projections known as microvilli, which form a surface similar to that of a brush called a brush border. As digested food passes through our small intestine, the brush border made up of millions of microvilli allows us to absorb nutrients into the bloodstream.

The brush border produces various enzymes (known as brush border enzymes) that break down larger nutrients into smaller molecules, which can then be easily absorbed into the bloodstream.

Source: Fassio, F., Facioni, M. S., & Guagnini, F. (2018). Lactose maldigestion, malabsorption, and intolerance: a comprehensive review with a focus on current management and future perspectives. Nutrients, 10(11), 1599.
In this respect, lactase (LPH) produced by our microvilli breaks down lactose into its constituent sugars, glucose (Glu) and galactose (Gal). These are then absorbed by microvilli into the bloodstream, where they can circulate around the body and be used by tissues for energy.
As infants, we produce high amounts of lactase, which enables us to digest and obtain energy from breast milk. As we grow up and start to eat solid foods, however, the majority of us stop producing lactase in significant amounts.
KEY POINTS
- Lactase is an enzyme produced by the small intestine that breaks down lactose.
- We produce high amounts of lactase as infant, enabling us to digest lactose in milk.
- Lactase production significantly declines after infancy.
What is lactase non-persistence?
As mentioned above, we produce high amounts of lactase as infants, which enables us to break down lactose in human breast milk when nursing.
During the weaning phase and in the first 10 years of life, however, lactase production rapidly drops off in about 2/3rds of the global population. This phenomenon is known as lactase non-persistence (LNP).
The majority of adults therefore produce little to no lactase, although this varies considerably between different populations and ethnicities. (As we’ll explain later, this is due to differences in ancestral milk-drinking habits, other evolutionary pressures, and ancient migration patterns, resulting in varying frequencies of genes that control lactase production in different populations). For example, virtually all of the Chinese population exhibits lactase non-persistence, compared to as little as 5% in Scandinavian countries.
In theory, lactase non-persistence ought to cause someone to be unable to digest lactose from cow’s milk and other dairy products as an adult. This is because the drastically reduced ability to produce lactase means that lactose from food is not broken down and instead passes undigested and unabsorbed through the small intestine. Intact lactose then accumulates in the colon, drawing in water and also acting as a substrate for bacteria which ferment lactose into gasses and short chain fatty acids (SCFAs). This can lead to unpleasant symptoms such as diarrhoea, bloating, nausea, and abdominal pain.

Source: Misselwitz, B., Butter, M., Verbeke, K., & Fox, M. R. (2019). Update on lactose malabsorption and intolerance: pathogenesis, diagnosis and clinical management. Gut, 68(11), 2080-2091.
In reality, the picture is a bit more complicated and lactase non-persistence often doesn’t cause any issues digesting lactose.
For example, a UK Biobank study suggested that 92% of people with lactase non-persistence still consumed cow’s milk. From a historical perspective, early Neolithic peoples (approximately 10,000 years ago) around Europe were almost exclusively lactase non-persistent, yet archaeological evidence suggests that milk consumption was widespread. More recently, milk consumption has increased more than 25-fold in recent decades in China, a population that is virtually entirely lactase non-persistent.
We elaborate upon the possible reasons that people with lactase non-persistence can still digest and tolerate lactose in a later section.
It is also worth noting that lactase non-persistence is not a disease or pathological condition, but merely a normal variation of human metabolism. In fact, as previously alluded to, the majority of us have lactase non-persistence.
KEY POINTS
- Lactase non-persistence describes a trait whereby humans stop producing lactase after infancy.
- Roughly 2/3rds of us have lactase non-persistence.
- Lactase non-persistence may lead to an inability to digest lactose in dairy products, although this is not always the case.
What is lactase persistence?
Whereas the majority of us stop producing lactase after infancy, about a third of the global population continues to produce high amounts of lactase well into adulthood. This trait is known as lactase persistence.

Source: Perino, A., Cabras, S., Obinu, D., & Sforza, L. C. (2009). Lactose intolerance: a non-allergic disorder often managed by allergologists. European annals of allergy and clinical immunology, 41(1), 3.
People with lactase persistence can therefore break down, digest and absorb high amounts of lactose in cow’s milk and other dairy products as adults.
Evolutionarily speaking, it is thought that this trait was strongly selected for as it enabled individuals to get nutrients from milk and provided a strong survival advantage, particularly in times of famine.
On this note, lactase persistence is more frequently seen in populations that were historically based on pastoral and agropastoral farming and relied more heavily on milk as a source of nutrition.

Source: Itan, Y., Jones, B. L., Ingram, C. J., Swallow, D. M., & Thomas, M. G. (2010). A worldwide correlation of lactase persistence phenotype and genotypes. BMC evolutionary biology, 10(1), 1-11.
For example, as shown in the diagram above, lactase persistence (LP) is very common in Northern European populations, with estimates suggesting that 89-96% of people in Britain and Scandinavia having the trait. By contrast, lactase persistence is very rare in East Asian (e.g. Han Chinese, Korean) populations.
We explain the evolutionary theories behind lactase persistence in a later section.
KEY POINTS
- Lactase persistence describes a trait whereby people continue to produce high amounts of lactase into adulthood.
- Lactase persistence can allow people to consume high amounts of lactose in dairy products.
How do genes influence lactase persistence / non-persistence?
Whether or not we produce lactase into adulthood is strongly determined by our genes.
The specific gene variants that give rise to lactase persistence vary according to our ancestry.

Source: Misselwitz, B., Butter, M., Verbeke, K., & Fox, M. R. (2019). Update on lactose malabsorption and intolerance: pathogenesis, diagnosis and clinical management. Gut, 68(11), 2080-2091.
- Eurasian populations
In Eurasian and some North and Central African populations, lactase persistence is caused by a single mutation near the LCT gene - the gene that carries the DNA instructions for making our lactase enzyme. (The next bit may be a bit confusing, so bear with us!)
This mutation or SNP (single nucleotide polymorphism), designated rs4988235 or 13.910:C>T, actually causes a C → T change in the DNA code of another gene, called MCM6 (minichromosone maintenance complex component 6).
The MCM6 gene, which lies close to the LCT gene, acts as an ‘enhancer’ - a region of DNA that can switch on other genes when bound by a specialised type of protein called a transcription factor.
Usually, after weaning, the LCT gene is “switched off” or “silenced”. This means that the DNA instructions for making the lactase enzyme are no longer read and followed, causing a dramatic fall in the production of lactase. This is what happens in lactase non-persistence.
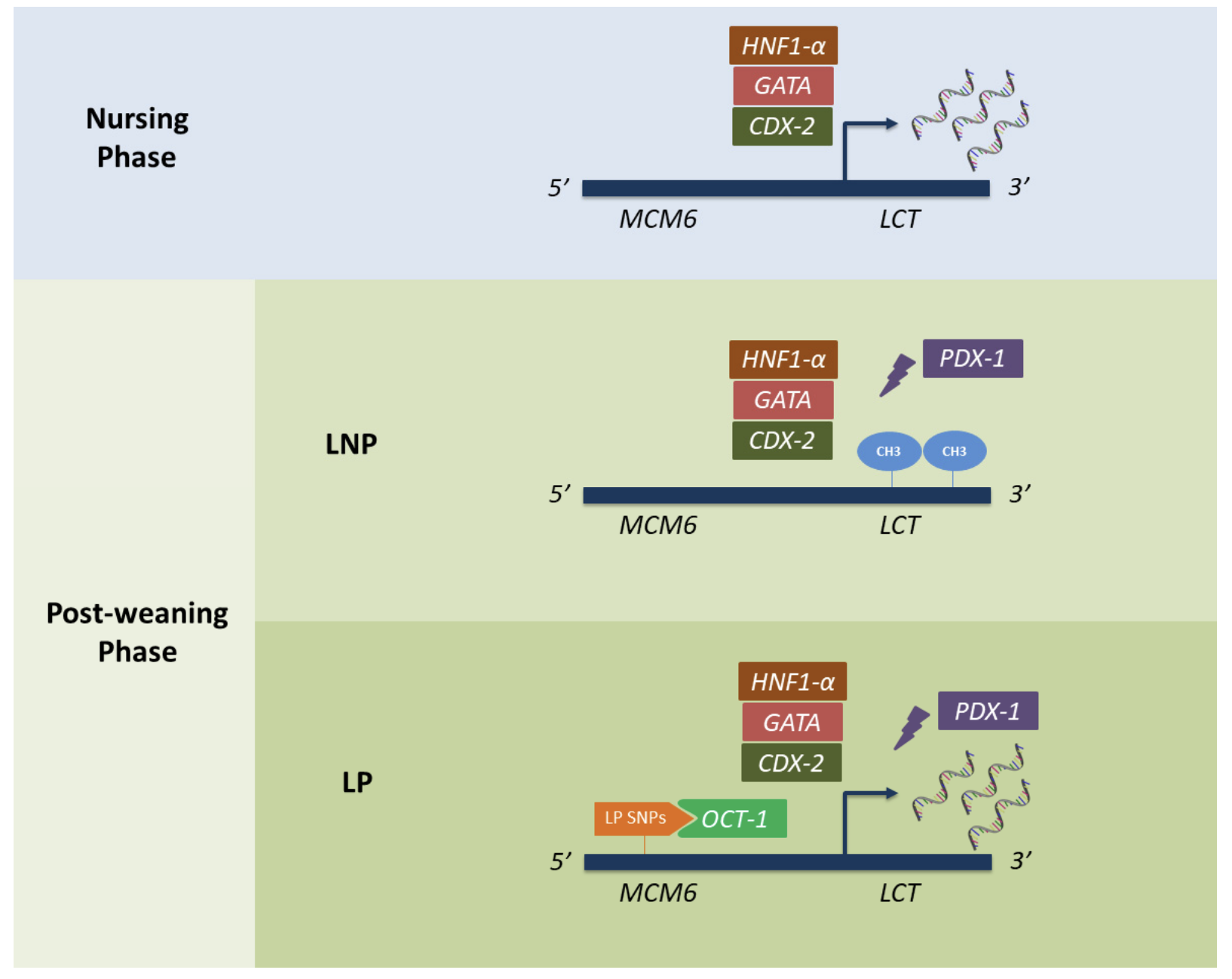
Source: Ségurel, L., & Bon, C. (2017). On the evolution of lactase persistence in humans. Annual review of genomics and human genetics, 18, 297-319.
The ‘T’ variant of the MCM6 gene (created by the rs4988235 SNP), however, results in a new binding site for a transcription factor that “switches on” the lactase (LCT) gene. The DNA instructions for making lactase therefore continue to be read and followed, resulting in the continued high production of lactase, even after weaning.
(Note that in your Lactose Tolerance trait, we refer to LCT gene variants to simplify things. In reality, the rs4988235 SNP occurs in the MCM6 gene, which is a separate gene, but it is close to the LCT gene and has a demonstrable impact on LCT gene expression.)
- How is lactase persistence inherited?
Lactase persistence is thought to be an autosomal dominant trait.
The term ‘dominant’ means that you only need to inherit one copy of the ‘T’ variant (rs4988235) of the MCM6/LCT gene (from either parent) to have lactase persistence. The term “autosomal” means that the ‘T’ variant is not located on a sex chromosome (i.e. X or Y chromosome).
Given this pattern of inheritance, you can belong to one of two possible groups:
- Lactase non-persistence (LNP) - CC genotype of MCM6 / LCT gene (rs4988235). We also call this group “genetically lactose intolerant”.
- Lactase persistence (LP) - CT and TT genotypes of MCM6/LCT gene (rs4988235).* We also call this group “genetically lactose tolerant”.
* In reality, there may be slight differences between the CT and TT genotypes in terms of lactase activity and ability to break down lactose under certain conditions. More specifically, some studies suggest that those with the CT genotype may have slightly lower lactase activity, which may increase the risk of symptoms of lactose intolerance (e.g. diarrhoea, bloating) during times of stress or illness.
- East African, Middle Eastern and other populations
Different mutations seem to underlie lactase persistence in populations from the Arabian peninsula and East Africa.
Whereas a single mutation / SNP (rs4988235) is responsible for lactase persistence in Eurasian populations, four key SNPs, all still within the MCM6 gene, have been identified in Middle Eastern and East African populations. These are:
- rs41525747 (13.907:C>G)
- rs41380347 (13.915:T>G)
- ss820486565 (14.009:T>G)
- rs145946881 (14.010:G>C)

Source: Anguita-Ruiz, A., Aguilera, C. M., & Gil, Á. (2020). Genetics of lactose intolerance: an updated review and online interactive world maps of phenotype and genotype frequencies. Nutrients, 12(9), 2689.
The frequency of these SNPs varies between different ancestral populations, as illustrated in the map above. For example, the rs145946881 SNP is most prevalent in East Africa, whereas the rs41380347 SNP is the main mutation that causes lactase persistence in camel herders from the Middle East. In Ethiopia and in the Beja people from Sudan, rs41525747 and ss820486565 are the main SNPs responsible for lactase persistence.
In other smaller populations, rarer mutations can cause lactase persistence. Overall, as of 2020, there have been 23 SNPs associated with lactase persistence. If you’re interested in reading more about the genetics of lactase persistence, this review in Nutrients is a good starting point.
Interestingly, these mutations seem to have arisen independently in different populations and then persisted in the gene pool of each population as they provided a survival advantage. As we’ll explain in the next section, this is a textbook example of what is known as “convergent evolution.”
(Note: Unfortunately, due to the absence of various lactase persistence SNPs on the microarray chip that analyses your DNA, we are only able to report on the rs4988235 SNP that governs lactase persistence in Eurasian, North African and Central African populations).
KEY POINTS
- Lactase persistence is determined by our genetics.
- The 'T' variant of the MCM6/LCT gene (rs4988235) causes lactase persistence in people of Eurasian ancestry.
- Inheriting one or two copies of the 'T' variant results in continued activation of the LCT gene (which encodes the lactase enzyme), meaning lactase production is not switched off after weaning during infancy.
- Four other SNPs in the MCM6/LCT gene are largely responsible for lactase persistence in East African and Middle Eastern populations.
How did genes for lactase persistence arise and spread in populations?
The rs4988235 (13.910:C>T) SNP, which is responsible for lactase persistence in Eurasian populations, has been identified in prehistoric skeletons from Spain, dating to about 5000 BC. Other genetic modelling techniques have suggested that this mutation arose in Eurasian populations sometime between 2,188 and 20,650 years ago.
The rs145946881 (14.010:G>C) SNP, responsible for lactase persistence in East African populations, is thought to have arisen 1,200 to 23,200 years ago. On a timescale of human evolution, these lactase-persistence mutations can be considered fairly recent.
After first arising, such mutations have increased rapidly in populations over the last 10,000 or so years. (This is illustrated in the graph below for the rs4988235 SNP). Moreover, it seems unlikely that such a rapid rise was due to genetic drift, the change in frequency of gene variants in a population due to random chance.

Source: Ségurel, L., & Bon, C. (2017). On the evolution of lactase persistence in humans. Annual review of genomics and human genetics, 18, 297-319.
This raises an obvious question: why did these lactase persistence mutations spread so rapidly?
The answer is due to natural selection. In the same way mutations/genes conferring resistance to malaria provided a strong survival advantage and were selected for in African populations where malaria poses a threat to life, the mutations/genes responsible for lactase persistence likely provided a strong survival advantage in populations that relied upon milk as a source of nutrition.
In fact, the selection coefficient for lactase persistence is estimated to be 0.04 - 0.05, meaning that it provided a survival advantage of 4-5% per generation. This makes these lactase persistence mutations some of the most strongly selected for in the human genome.
Arguably the most widely-supported theory for explaining the natural selection and spread of lactase persistence is known as the cultural-historical hypothesis.
Under this theory, in the early Neolithic period (about 10,500 years ago), humans shifted from a hunter-gatherer lifestyle to an agricultural or pastoralist lifestyle involving the domestication of animals and the consumption of their milk. As this practice of animal domestication and milk drinking arose and spread across the globe, people with lactase persistence genes would have been better able to digest lactose and obtain nutrients from milk as an adult. They would have also avoided the negative gastrointestinal symptoms of being unable to digest lactose, such as diarrhoea. Consequently, such individuals would have been better able to survive, reproduce, and pass on these lactase persistence genes to the next generation.
Over time, this process of positive selection (the spread of beneficial gene variants) would have led to the rapid rise in frequency of lactase-persistence genes in agricultural/pastoralist populations that depended on milk. On this note, there seems to be a positive correlation between whether a population historically adopted a pastoralist lifestyle and depended on milk, and the frequency of lactase persistence (as illustrated in the graph below).

Source: Ségurel, L., & Bon, C. (2017). On the evolution of lactase persistence in humans. Annual review of genomics and human genetics, 18, 297-319.
It’s worth pointing out that the cultural-historical hypothesis of lactase persistence is subject to scientific debate and faces some puzzling findings. For example, some populations heavily dependent on milk as a source of nutrition do not appear to have high frequencies of lactase persistence (e.g. long-term Kazakh and Mongol herders). The obverse is also true, some hunter-gatherer populations (e.g. the Hadza and Yaaku of east Africa) have high frequencies of lactase persistence genes, despite virtually zero milk consumption. Interested readers are directed to this excellent review of the evolution of lactase persistence, published in the Annual Review of Genetics and Human Genetics.
- Why were lactase persistence genes selected for?
There are several theories of how lactase persistence and the ability to digest lactose into adulthood would have been advantageous and promoted survival. These include:
- Lactase persistence allows individuals to rely on milk and dairy products as a lifelong source of nutrients and fluid, not just during infancy. This would have been particularly advantageous during times of famine.
- Lactase persistence made individuals less likely to suffer from diarrhoea and other gastrointestinal symptoms when consuming lactose. This would have been particularly important in agricultural/pastoralist populations, as rearing animals would increase the risk of zoonotic infections, and such infections would be exacerbated by symptoms such as diarrhoea.
- Lactase persistence allows the consumption of lactose, which increases the absorption of calcium. As vitamin D is also required for calcium absorption, this benefit of lactase persistence may have been particularly important at high latitudes (e.g. in Northern Europe), as it could mitigate against reduced sunlight exposure and Vitamin D production in these regions.
- Lactase persistence supports the consumption of milk, which may accelerate physical growth and reproductive development through its effects on hormones such as insulin-like growth factor I.
As mentioned earlier, the mutations that cause lactase persistence seem to have arisen and been selected for independently in different populations as they adopted pastoralist lifestyles and relied upon milk as an energy source.
For example, the rs4988235 (13.910:C>T) SNP in Eurasian populations is thought to have been selected for after cattle domestication began and spread from Anatolia around 10,500 years ago. By contrast, cattle farming did not spread south of the Sahara until about 4,500 years ago. This could explain why the rs4988235 SNP is largely absent in East African populations, with the rs145946881 (14.010:G>C) SNP responsible for lactase persistence in East African populations arising later.
Lactase persistence is therefore a good example of convergent evolution - the process by which unrelated populations independently find their way to the same adaptation to the same environmental pressures. In this case, the strong survival advantages of being able to digest lactose and consume milk led to the selection of lactase persistence in different populations across the globe at different times as they began domesticating and consuming the milk of cows, sheep, goats, horses, donkeys, and camels.
KEY POINTS
- The mutations in the MCM6/LCT gene responsible for lactase persistence likely arose and spread in the early Neolithic period (about 10,500 years ago) as humans transitioned to agriculture and began domesticating milk-producing animals.
- Broadly speaking, populations with an ancestral history of pastoralism/animal-farming have higher frequencies of lactase-persistence mutations.
- The genes responsible for lactase persistence likely conferred a strong survival advantage as they allowed individuals carrying these mutations to consume milk and use it as a lifelong source of nutrition. These mutations were therefore selected for and increased frequency in populations over time through a process of natural selection.
- The mutations for lactase persistence likely arose and spread independently in different populations - an example of convergent evolution.
What is lactose intolerance?
Lactose intolerance is a condition characterised by unpleasant symptoms, such as diarrhoea, bloating, flatulence, and abdominal pain, after consuming lactose.

These symptoms are caused by the inability to digest and break down lactose in the small intestine, which leads to the accumulation of lactose in the colon. As undigested lactose exerts an ‘osmotic load’, it draws water and electrolytes into the lumen of the colon, giving rise to diarrhoea.
Gut bacteria residing in the colon also start to ferment undigested lactose, converting it into short chain fatty acids (SCFAs) and gases (including carbon dioxide, methane, and hydrogen). The generation of these metabolites is thought to lead to symptoms such as bloating, abdominal discomfort, and flatulence.

Source: Misselwitz, B., Butter, M., Verbeke, K., & Fox, M. R. (2019). Update on lactose malabsorption and intolerance: pathogenesis, diagnosis and clinical management. Gut, 68(11), 2080-2091.
Interestingly, the hydrogen gas produced when colonic bacteria ferment undigested lactose is detectable in exhaled air. Accordingly, one of the diagnostic tests for lactose intolerance is a hydrogen breath test. This involves getting a patient to consume a lactose solution and then exhale into a balloon-like bag at regular intervals over a few hours. An increase in exhaled hydrogen of >20 ppm after 3 hours is considered diagnostic of lactose intolerance.
Other more specialised tests, involving 13-C lactose and detection of carbon dioxide are also available in specialist laboratories.
It is important to note that lactose intolerance is not an allergy. Allergies involve a specific immune response and typically lead to more rapidly-onset symptoms such as rash, itchiness, and wheezing.
You can read more about lactose intolerance on the NHS website.
KEY POINTS
- Lactose intolerance is a condition whereby consuming lactose leads to unpleasant symptoms such as diarrhoea, bloating, flatulence, and abdominal pain.
- Symptoms of lactose intolerance occur when undigested lactose accumulates in the colon, causing the entry of fluid and the build-up of acids and gases as lactose is fermented by gut bacteria.
- Lactose intolerance can be diagnosed using a hydrogen breath test.
What is the difference between lactase non-persistence and lactose intolerance?
Lactase non-persistence describes the trait whereby someone stops producing lactase in high amounts after infancy. As mentioned previously, this trait is strongly determined by variants of the MCM6/LCT gene.
By contrast, lactose intolerance describes the condition whereby consuming lactose gives rise to unpleasant gastrointestinal symptoms (e.g. bloating, diarrhoea). Someone who has lactase non-persistence is more likely to have lactose intolerance, but the likelihood of experiencing symptoms is also dependent on several other factors, including the amount of lactose consumed, gut microbiome composition, and the presence of anxiety disorders (see image below).

Source: Misselwitz, B., Butter, M., Verbeke, K., & Fox, M. R. (2019). Update on lactose malabsorption and intolerance: pathogenesis, diagnosis and clinical management. Gut, 68(11), 2080-2091.
Importantly, many people who have lactase non-persistence do not have lactose intolerance and can still consume lactose without any unpleasant symptoms. For example, a UK Biobank study of 500,000 subjects found that 92% of those with lactase non-persistence (based on MCM6/LCT gene variants) still regularly consumed cow’s milk containing lactose.
KEY POINTS
- Lactose intolerance describes the negative symptoms after consuming lactose, whereas lactase non-persistence describes the sharp reduction in lactase production after infancy.
- Lactase non-persistence does not always lead to lactose intolerance. Many people with lactase non-persistence can still consume and tolerate lactose.
- The likelihood of having symptoms of lactose intolerance depends on several factors, such as the make-up of your gut microbiome, amount of lactose consumed, having psychiatric comorbidities.
My genetics suggest I have lactase non-persistence, but I can still tolerate lactose. Why?
As explained previously, many people with lactase non-persistence can still consume lactose without any unpleasant symptoms. There are several possible reasons for this, including the presence of gut bacteria that enable digestion of lactose, the presence of other genes that facilitate lactose digestion and absorption, and other dietary factors (such as the dose of lactose).
Lactose digestion and the gut microbiota
The gut microbiota refers to the collection of 10-100 trillion bacteria, viruses, and other microorganisms that colonise our gastrointestinal tract. The composition of our gut microbiota can have a strong effect on whether or not we can tolerate lactose in our diet.
A study of 1,126 pairs of UK twins found that those with lactase non-persistence (based on MCM6/LCT genotype) harboured higher levels of Bifidobacteria in their colon. This gut bacteria can break down and ferment lactose. Higher amounts of Bifidobacteria may therefore enable the digestion of lactose in those with lactase non-persistence, despite the absence of the lactase enzyme in the small intestine.
Of course, the metabolites of lactose fermentation include short chain fatty acids (SCFAs) and gases (e.g. hydrogen), which can lead to unpleasant symptoms of lactose intolerance. However, if only small amounts of lactose pass into the colon, or lactose takes longer to pass into the colon (known as increased transit time), then the likelihood of these symptoms decreases. This may explain why some people with lactase non-persistence can tolerate low amounts of lactose, but may experience unpleasant symptoms with higher doses.
The regular consumption of dairy products may also engender other changes in gut microbiota composition that enable digestion of lactose. For example, one study found that gradually increasing lactose consumption over a period of 6-12 weeks led to a lessening of unpleasant symptoms. Similarly, changes in gut microbiota during pregnancy may also account for reduced lactose intolerance symptoms experienced by some pregnant women.
Further evidence for the role of gut microbiota in lactose digestion comes from studies in which subjects consumed probiotic foods. For example, one small study gave subjects a probiotic yoghurt containing Lactobacillus.casei Shirota and Bifidobacterium.breve bacteria. After 4 weeks, symptoms of lactose intolerance decreased significantly, as did the amount of hydrogen exhaled by subjects on breath testing. Moreover, these reductions continued for 3 months after subjects stopped eating the probiotic yoghurts.
A good review of the role of gut microbiota in the digestion and tolerance of lactose is in this edition of Nutrients.
KEY POINTS
- The presence of certain gut bacteria can enable the digestion of lactose despite having lactase non-persistence.




.png)
